Protocol of Daphnia culture
Daphnia as live feed for zebrafish
Nutritional quality of Daphnia
 Live zooplankton such as Daphnia is an essential food source for many species of fish such as zebrafish, freshwater. Daphnia can raise advanced juvenile stages as well as adults, they are a very rich source of food (Koivisto, 1995). It is important to mention that daphnia, as filtering animals, derives their exceptional nutritional value from what they eat, namely very high-quality phytoplankton (Phyto-Feast®, RGcomplete®, etc.).
Live zooplankton such as Daphnia is an essential food source for many species of fish such as zebrafish, freshwater. Daphnia can raise advanced juvenile stages as well as adults, they are a very rich source of food (Koivisto, 1995). It is important to mention that daphnia, as filtering animals, derives their exceptional nutritional value from what they eat, namely very high-quality phytoplankton (Phyto-Feast®, RGcomplete®, etc.).
 These highly concentrated phytoplankton solutions are mixtures of 6 selected microalgae and provided with a non-negligible concentration of astaxanthin dosed very precisely to meet the nutritional needs of the inhabitants of our aquariums. In addition, these microalgae are preserved, quiescent, and so unable to divide themselves, thus ensuring their nutritional value over a long period (6 months), while avoiding a bloom of algae in the aquarium.
These highly concentrated phytoplankton solutions are mixtures of 6 selected microalgae and provided with a non-negligible concentration of astaxanthin dosed very precisely to meet the nutritional needs of the inhabitants of our aquariums. In addition, these microalgae are preserved, quiescent, and so unable to divide themselves, thus ensuring their nutritional value over a long period (6 months), while avoiding a bloom of algae in the aquarium.
Because of this rich diet and their metabolism, daphnia produces carotenoids that will strongly help the survival of the fish that eat them. Indeed, these carotenoids produced by daphnia act as anti-oxidants and promote resistance to low oxygen levels in fish eggs and larvae (Dave, 1989). In addition, daphnias are larger organisms than rotifers. Thus, when juveniles are no longer sufficient to feed on juvenile fish, Daphnias are excellent sources of nutrition (He and al., 2001).
Finally, these daphnias live in the water column in their natural environment and are thus mobile. This makes them easy to target for fish larvae. Females lay hundreds of eggs during their entire lives. In addition, they perform a cleaning of the water column by consuming detritus, invasive algae, and phytoplankton in aquariums.
1. Daphnia culture protocol
This protocol presents the equipment and the steps required for the production of zooplankton. It uses small-scale glassware to adapt the zooplankton culture to the use and weekly needs of live feed for our aquariums.
1.1. Equipment and specification for Daphnia culture
- Compact Culture System (SCC, APBreed),
- 120μm and 1mm mesh size sieve (HOBBY)
- Artificial or natural lighting at 14L/10N,
- Ideally, culture water is prepared from demineralized water and commercial mineral salts (Mineral salts, JBL.com, Planktovie). At the rate of 9g of mineral salts for 15 liters of distilled water.
- Airflow: 1.5-2 LPM, it is provided by the SCC filtration system and an air pump (EHEIM),
- Salinity: 0 ppt,
- Temperature: 20 to 22°C (EHEIM heater),
- pH: 7-8,
- Ammonia (NH3): maximum 1mg/L,
- Food: RG complete® (APBreed)
- Quantity of food: 1mL of RG complete® to produce 500 daphnia per day on average (see: Scientific study report: Lefranc and al., 2018, Pauw et al., 1981),
- Feeding Frequency: Depending on the amount of daphnia to be harvested each day, every 2 to 4 hours on average, the RG complete® is delivered per peristaltic pump.
- Harvest: 20% -30% daily
1.2. Culturing
As a first step, the culture must be prepared with distilled water or “dechlorinated” tap water by aeration. This using an air pump and a “bubbler”. Add to this water 9g of JBL mineral salts in 15 liters of distilled water, equivalent to a carbonate hardness of 9° KH (German unit). Then install a mixer and water aerator system such as an air pump and a “bubbler”. Let it air for 12 minutes. Immerse the bottle containing daphnia in the culture tank without opening the bottle. This is to acclimatize daphnia to the temperature of their new environment.
After 30 minutes of acclimation, pour the Daphnia into a tank containing the culture medium previously prepared. Feed the daphnia with a solution of RG complete diluted in culture medium so that the algae mix well with the culture of daphnia.
1.3. Daphnia culture Maintenance
Maintaining a culture of daphnia consists mainly of renewing part of the water in the culture medium, cleaning the CSC filter mass and adjusting the amount of nutrients delivered to daphnia from day to day.
For this, we install a 120-micron sieve in a crystallizer which will allow sifting the water of the medium to be renewed by harvesting or concentrating the daphnia of the tank. The sieve is previously semi-immersed in culture medium so that the sieved daphnia remains in suspension. We sift 33% of the total volume of the crop.
Then, readjust the water level of the culture medium with new culture water. Then, if the culture medium is clear, it is possible to observe daphnia at the bottom of the tank, this is the sign that we must feed more culture microalgae (RGcomplete ® for example).
1.4. Harvesting daphnia
The harvest of Daphnia to feed the juveniles and adult fish of the aquariums is done with 2 sieves. A first mesh size sieve 1mm will trap adult Daphnia. A second 120 μm mesh size sieve, positioned below the first sieve, will trap young Daphnia. The adult daphnia is suspended again in a beaker and then given to the aquarium fish. The young daphnia is concentrated in another beaker and put back into the daphnia culture tank.
This will help maintain a culture of young daphnia while providing large live prey for our aquarium fish. If a higher density of daphnia is needed, add an intermediate sieve (600 μm) which will be used to feed the fish until the density of daphnia increases. Older daphnia is larger, producing more eggs at each egg-laying than smaller individuals. Thus, by keeping the larger individuals in the culture tank, this will significantly increase the reproductive rate of the Daphnia population.
1.5. Density estimation of the Daphnia population
The density of our population of Daphnia is estimated using the following equipment:
- A binocular loupe,
- A 50 ml beaker,
- A solution of Lugol diluted to 10%,
- Plasticized sheet with counting area with known area
Before any sampling of the culture of daphnia, thoroughly homogenize the medium using a beaker of 50 ml to 1 liter for example. Next, take 20 ml of the culture and fix them to Lugol (500 μl) at the same time, pour both solutions directly into the 50 ml beaker. Place the attached sample on the plasticized count sheet under the magnifying glass. Do not stir the sample as this will concentrate the organisms in the center of the beaker (centripetal force). Then let stand 30 seconds.
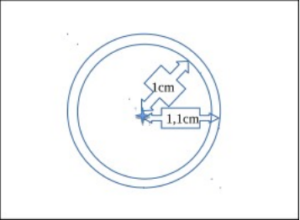
Then, perform a manual count of the daphnia present inside the 2 count circles but not touching the outermost circle (figure No. 1 of the circles and counting situation). Thus you determine the density of individuals of your population in 10 ml of culture. These 10 ml correspond to the volume present in the water column above the count circle. From this data, you can extrapolate to the whole culture volume to find the total density of daphnia in the tank.
FIG. 1: Daphnia counting cell with a binocular magnifying glass, which makes it possible to count the individuals present in 10 ml of culture medium from a 20 ml sample.
Tips for a successful culture :
If you observe too high ammonia levels, especially after each weekend when maintenance is not carried out, increased maintenance must be done. It is advisable to reduce the ammonia content considerably by renewing more than 33% of the volume of culture, ie 50% to 66% if necessary.
It is recommended to reduce the amount of nutrients delivered by the peristaltic pump during the weekend so that the filtration by the animals is complete and environmental hygiene is maintained (Ebert, 2005). Then, if the molts accumulate in the bottom of the tank and are not sufficiently removed by the filtering mass of the SCC, you can siphon the bottom of the tank with a pipette 10 ml graduated and a pro-pipette. This at a rate of once a week.
You are ready, it’s up to you!
Bibliographie
http://aquariophilie.wikibis.com/daphnie.php
http://cerclaqua09.cerclaqua.com/eaudouce/articles/daphnies.php
http://aquabulle.forumperso.com/t135-4-eme-partie-l-elevage-des-daphnies
https://www-ncbi-nlm-nih-gov.www.ezp.biu-montpellier.fr/books/NBK2042/
– Dave, G., 1989. Experiences with wastewater-cultured Daphnia in the start-feeding of rainbow trout (Salmo gairdneri). Aquaculture 79, 337–343. https://doi.org/10.1016/0044-8486(89)90475-4
– Ebert, D., 2005. Ecology, Epidemiology, and Evolution of Parasitism in Daphnia. Natl. Cent. Biotechnol. Inf. 1–30.
– He, Z.H., Qin, J.G., Wang, Y., Jiang, H., Wen, Z., 2001. Biology of Moina mongolica (Moinidae, Cladocera) and perspective as live food for marine fish larvae. Hydrobiologia 457, 25–37.
– Koivisto, S., 1995. Is Daphnia magna an ecologically representative zooplankton species in toxicity tests? Environ. Pollut. 90, 263–267. https://doi.org/10.1016/0269-7491(95)00029-Q
– Pauw, N.D., Laureys, P., Morales, J., 1981. Mass cultivation of Daphnia magna Straus on
ricebran. Aquaculture 25, 141–152. https://doi.org/10.1016/0044-8486(81)90176-9

